Welcome To Our Company
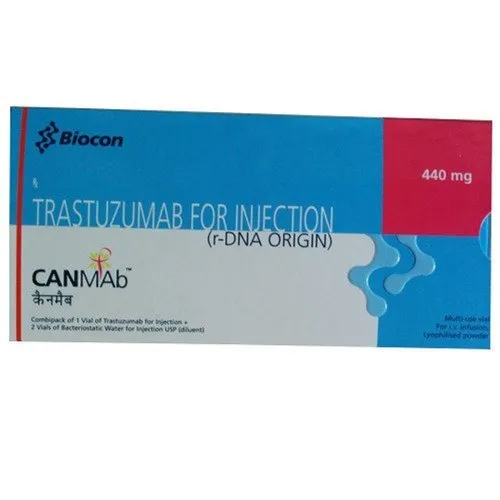
Canmab 440 mg Injection
9000 INR/Unit
Product Details:
- Origin India
- Dosage Form Tablet
- Fermentation Smell Normal Smell
- Storage Instructions Cold and Dry place
- Shelf Life 12-24 Months
- Click to view more
X
Canmab 440 mg Injection Price And Quantity
- 10 Unit
- 9000 INR/Unit
Canmab 440 mg Injection Product Specifications
- Tablet
- India
- Cold and Dry place
- 12-24 Months
- Normal Smell
Canmab 440 mg Injection Trade Information
- Cash in Advance (CID)
- 5000 Unit Per Month
- 2-7 Days
- All India
Product Description
Canmab 440 mg Injection is a highly effective medication used to treat breast cancer, stomach and lung cancer, and other types of tumors. This medication is formulated in India and is available in a tablet dosage form. The fermentation smell of the medicine is normal. The shelf life of the product is 12-24 months, and it should be stored in a cold and dry place. Canmab 440 mg Injection contains Trastuzumab, which is a monoclonal antibody that works by inhibiting the growth of cancer cells. This medication is recommended for patients who have been diagnosed with HER2-positive cancer.
FAQs of Canmab 440 mg Injection:
Q: How should Canmab 440 mg Injection be administered?
A: Canmab 440 mg Injection should be administered under the supervision of a healthcare professional. The medication is given through an intravenous (IV) infusion.Q: What are the side effects of Canmab 440 mg Injection?
A: The common side effects of Canmab 440 mg Injection include fever, chills, headache, nausea, vomiting, diarrhea, and weakness. In rare cases, it can cause serious side effects such as heart problems, allergic reactions, and lung issues.Q: Can Canmab 440 mg Injection be used during pregnancy?
A: No, Canmab 440 mg Injection should not be used during pregnancy as it can harm the developing fetus. It is recommended to use effective birth control while taking this medication.Q: Is it safe to drive while taking Canmab 440 mg Injection?
A: Canmab 440 mg Injection may cause dizziness and fatigue, which can affect your ability to drive. It is advisable to avoid driving or operating heavy machinery until you know how the medication affects you.Q: What should I do if I miss a dose of Canmab 440 mg Injection?
A: If you miss a dose of Canmab 440 mg Injection, contact your healthcare provider immediately. They will advise you on the appropriate course of action. It is important not to double the dose to make up for the missed one.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email